Clinical trials rely on high quality data to answer crucial questions around the patient’s condition, the benefits of the treatment, and its potential side effects. When this data is collected from participants, the method of collection makes a difference.
Traditionally, paper-based patient outcomes assessments such as diaries and surveys were used to find the answers. However, this system is prone to inaccuracies including missing data, poor data quality due to retrospective data entry (“parking lot syndrome”), patients not following instructions on a paper form, errors in transcribing paper-based data into an electronic system, and the time to enter and reconcile data between the paper and electronic systems. Electronic patient reported outcomes (ePRO) has since gained prominence over the past two decades—alleviating paper-based burdens while providing reliable, accurate, and secure data collection methods. The benefits of electronic collection not only apply to Patient Reported Outcomes (PRO) but to Clinical Outcome Assessments (COA) in general.
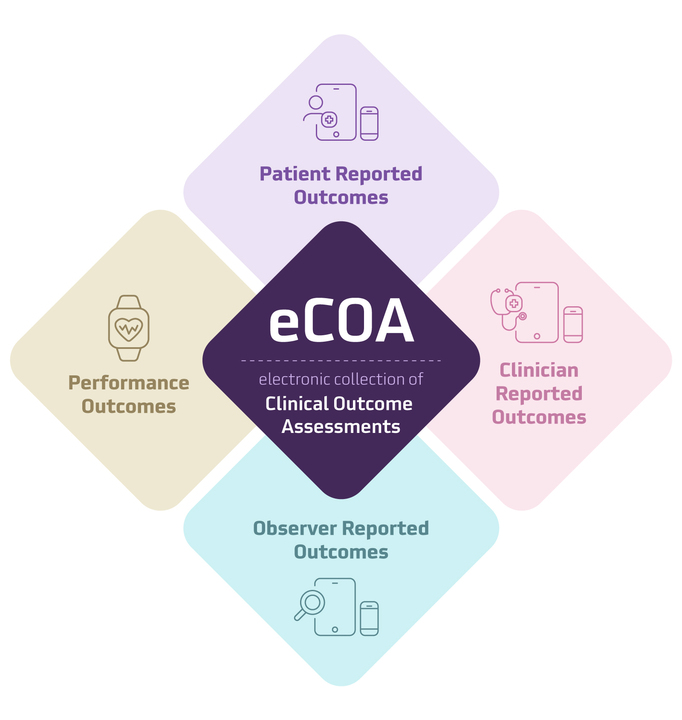
What is eCOA?
eCOA is the electronic collection of Clinical Outcome Assessments. At its core, eCOA focuses on improving data accuracy while prioritizing ease for patients and sites. It empowers clinicians, patients, and caregivers to report outcomes digitally, leaving behind the burden and error-prone nature of paper data collection. eCOA can include ePRO, as well as electronic clinician reported outcomes (eClinRO), electronic observer reported outcomes (eObsRO) and electronic performance outcomes (ePerfO).
Looking at ePRO specifically, it is a set of tools to collect outcome measures related to a patient’s experiences and health during a clinical trial, encompassing:
- Physical symptoms: Tangible symptoms such as pain, itch, or digestive problems.
- Ability to function: Ability to perform daily activities such as walking up stairs.
- Quality of Life: A broad concept covering many aspects of a patient’s health and well being.
It is especially useful in therapeutic areas that focus on understanding patient experiences and health outcomes, such as oncology, dermatology, and gastroenterology. Note that ePRO is not designed to capture laboratory measures like vital signs, bloodwork, or tissue samples.
How eCOA enables high-quality data collection
Several data collection methods are used—whether through provisioned or bring-your-own mobile data collection devices—to gather clinician-reported, patient-reported, and observer-reported outcomes. Some assessments that are collected include diaries, indication specific and quality-of-life questionnaires, and even wearables like Fitbit and activity monitors for performance outcomes.
With eCOA, the questionnaire data collection process is improved through:
- Better patient reporting
For patients, eCOA brings the convenience of entering data electronically wherever they are, minimizing recall errors and fostering sustained participation in clinical trials. Reminders for data submission help patients enter their data in real-time and can prevent data from being discarded due to inaccuracies. An early and pivotal eCOA study revealed that electronic patient outcomes reporting achieved 94% patient compliance, compared to the 11% compliance observed with paper-based methods. Since then, on average, studies using paper tend to achieve approximately 50% on-time reporting compliance. - Real-time data and insights
Site and sponsor teams get immediate visibility to patient information and data entry, which enables faster decision-making and interventions when necessary. The real-time data entry also eliminates errors associated with transcribing paper-based data into an electronic system. - Data privacy and compliance
eCOA platforms use encrypted channels to transmit patient data to study databases, protecting confidentiality and providing data protection while adhering to regulatory standards. The real-time visibility into outcomes data allows sponsors to respond promptly to data gaps and compliance issues, promoting data accuracy throughout the trial.
 Subsequently, the data collected and stored in an eCOA solution can be seamlessly integrated with other eClinical technologies like Interactive Response Technology (IRT) or electronic data capture (EDC) systems—reducing data re-entry. Study teams then have better data integrity and a clean database ready for analysis. The real-time visibility also allows them to proactively monitor trial progress, making sure that the data collected is compliant before submission.
Subsequently, the data collected and stored in an eCOA solution can be seamlessly integrated with other eClinical technologies like Interactive Response Technology (IRT) or electronic data capture (EDC) systems—reducing data re-entry. Study teams then have better data integrity and a clean database ready for analysis. The real-time visibility also allows them to proactively monitor trial progress, making sure that the data collected is compliant before submission.
Increasing the chances of trial success
Today’s clinical trials require resilience and demand efficiency, speed, and accuracy. eCOA has the potential to be an important tool for a more streamlined data collection process and reduced stakeholder burden. Like any technology, eCOA will be most effective at improving reporting compliance and data accuracy when the product is designed with the user at the center, with patients, sites, and sponsors providing input into the product design so the technology is usable and intuitive. What’s more, strong processes and purpose-built services surrounding eCOA delivery and maintenance are critical to realize its promise.
To deliver on the potential for eCOA to streamline and improve accurate data collection, Suvoda has centered patients in its design process. Suvoda eCOA is a powerful, user-friendly solution that provides rapid and efficient implementation, is delivered on a single platform with IRT and eConsent, and seamlessly integrates with other third-party clinical trial technologies.
Discover how Suvoda eCOA can bring you closer to more efficient, effective, and patient-centered clinical trials.
