A flexible eClinical ecosystem to give you full command over mission-critical, time-sensitive patient interactions.
Clinical trials are becoming increasingly complex, and the pressures to complete them faster are intensifying. Purpose-built trial management technologies are an essential tool to manage through complexity efficiently. Sponsors, sites, and patients need systems that work together seamlessly, integrate easily with other tools, can be updated with precision, and are efficiently deployed.
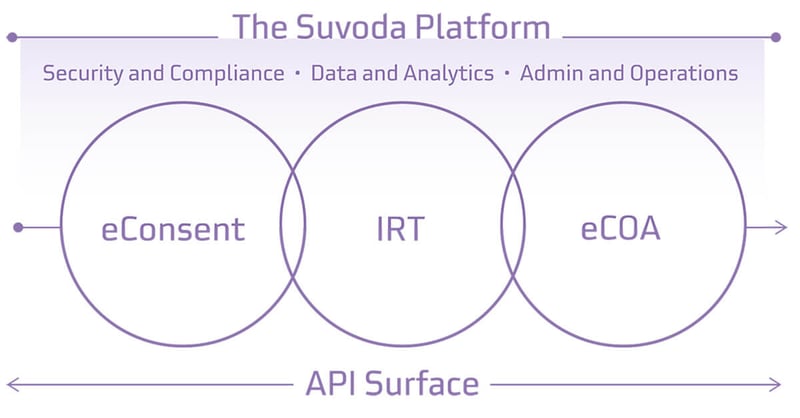
Suvoda’s “practical innovation” design philosophy puts the user at the center and leads to technology tools that solve problems elegantly, are practical for today, and are ready for whatever comes tomorrow. Our ecosystem of eConsent, IRT, and eCOA, all built on a single software platform, empowers sponsors, sites, and patients to take command over the patient experience throughout the trial.
Our platform delivers:
- Seamless clinical trial experience across eConsent, IRT, and eCOA
- Improved data workflow and reduced integrations
- Customer trial standardization and updates without disruption
- Rapid deployment, even for the most complex trials
Please share your contact information:
Schedule a Demo with our Expert:
Three clinical trial products built on one platform minimizes friction across the user experience for sponsors, CROs, sites, and patients.
Suvoda products - eConsent, IRT, and eCOA - operate on a single software platform. That means products are fully integrated from the outset. The shared backend software infrastructure and intuitive design create an easy-to-use workflow and more secure data sharing across the clinical trial journey - for patients, sites, and sponsors.
Platform features:
- Fully integrated product suite with shared user interface across eConsent, IRT, and eCOA
- Single sign on
- User workflow moves smoothly from one tool to the next, without having to switch systems
- Seamless workflow, all centered around the patient