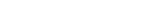IRT
Trusted experts
of services teams have complex trial experience
Average UAT defects
targeting ≤5 for moderate
& cosmetic defects
0n time delivery
for customer acceptance
A centralized control center for patient and drug logistics, designed to harness the complexities of clinical trials and help advance human health.
Managing clinical trial randomization and supply logistics can be challenging, especially with the complexities of modern clinical trials. Hybrid trial approaches, tight timelines, and the need for mid-study adjustments mean that sponsors and sites need precise and powerful systems to keep trials running efficiently and on-time.
From initial setup to mid-study adjustments to study completion, Suvoda Interactive Response Technology (IRT) gets the right drug to the right patient at the right time. Sometimes known as Randomization and Trial Supply Management (RTSM), this software has advanced capabilities that integrate seamlessly with existing clinical workflows and eClinical systems.
Now with Sofia, Suvoda’s safe and secure AI assistant, drug supply managers can have efficient access to vital information through an intuitive chat interface, reducing what were once multi-screen, multi-click processes to simple conversations. Sofia is currently in the early adopter stage. To learn more about how Sofia works, please see our FAQs or download the Sofia data sheet.
Learn more about Sofia Early Adopter Program
Suvoda IRT keeps trials running smoothly and supports sites, sponsors, and CROs to:
- Manage all clinical trial workflows efficiently, with data that is shared across systems
- Meet exact RTSM (trial randomization and drug supply management) needs with extensive configurability and customizability
- More simply conduct patient visits and drug dispensation with an intuitive interface and user-centric design
- Access robust, real-time reporting for enhanced trial oversight and decision-making
Clinical trial complexity, meet our rich RTSM functionality. Now you can address unique and changing needs—and hit tight timelines.
A robust foundation of essential core IRT / RTSM features, each focused on meeting the requirements of common protocols in decentralized, patient-centric trials. The tools to create new, client-custom functionality to support clinical research of increasingly novel, life-changing therapeutics. All developed as modular, easy-to-assemble building blocks. So our services teams can quickly launch systems that embrace the highest levels of protocol, operational, logistical, technical, and cultural complexity.
What’s more, the Suvoda IRT system also includes extensive configurability options. It’s how we enable study teams to adjust system functionality over the lifespan of a trial to meet ever-changing demands. Because we know that no two of our clients’ trials start out exactly alike. We also know trials need to evolve, with mid-project course corrections to respond to the specific hurdles they encounter or insights they collect. And they all need to do so with speed and ease.
Trial logistics
-
Roles, permissions, and blinding management
-
Study and site administration
-
Cohort/stage/phase management
-
Dynamic cohort and dose management
-
Additional and dynamic visit schedule
-
Embedded, intuitive Sofia AI assistant
Patient logistics
-
Subject management
-
Adaptive replacement and randomization
-
Cross-over and re-treatment
-
Open-label extension
-
Dose calculation
-
Subject roll-over
-
Dose modification and interruption
Drug logistics
-
Drug dispensing management
-
Drug supply management
-
Drug accountability, reconciliation, and returns/destruction management
-
Temperature excursion management
-
Controlled substance management
-
Variable drug sourcing
-
Central pharmacy
-
Direct-to-patient shipping
-
Robust supply strategy management
-
Fast access to drug supply data and visualizations with Sofia AI assistant
-
Drug supply forecasting
Built to embrace the unknown. Because your IRT should be as ready for change as you are.
The scientific process isn’t static. And neither is your trial. To collect the richest data, protocols must be amended as new information is obtained. That’s why we designed the Suvoda IRT to adapt mid-study with ease.
Commonly-needed additions, modifications, and corrections to study, site, drug management, and administrative functions can be made by permissioned users within our system after go-live. This puts control in your hands and reduces the time and costs associated with change orders. And if more significant updates are required, our modular architecture makes it a simple and speedy process for your dedicated Suvoda project team to add in new IRT features.
A user-friendly IRT/RTSM experience eases decisive action for users of all experience levels.
We find that people are more likely to work at their best when they’re using the tools they like best.
That’s why, no matter the level of experience with clinical trial technology—from dedicated pros to more occasional site users, and even home caregivers—you’ll find interactions with the Suvoda IRT are universally simple. It’s the result of a minimalist look and feel, intuitive navigation, and patient-centric workflow. And it’s all intended to drive focus on the immediate task at hand.
Tasks are now even faster and easier with Sofia, Suvoda’s AI assistant. Users can have the information they need at their fingertips just by asking Sofia questions. They can access trial data, look up user manual topics, compare depot inventories, review drug lot releases, create tables and graphs, and more, all while maintaining the study blind and with assurance that the answers are accurate. To learn more about how Sofia works, please see our FAQs.
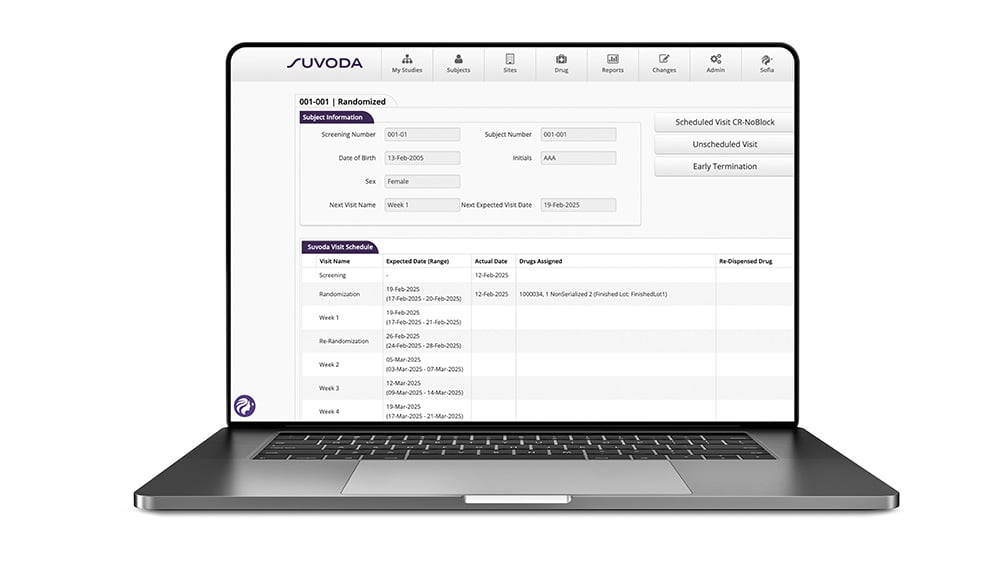
See the way to long-term trial performance, in real time, with pre-set and ad-hoc reports and advanced analytics.
Suvoda IRT pre-set and ad-hoc reports give you up-to-the-minute visualizations of data points, KPIs, and trends on the subjects, sites, drugs, and depots within a given study. It’s how you’ll always know where you stand—and what needs your attention.
Why such robust reporting? To give you the intelligence you need to make better decisions in executing on today’s trials—and planning for tomorrow’s.
FEATURED IRT MODULES

Take command of the cold supply chain
Gain control in a world of decentralized trials
Gain control in a world of decentralized trials
IRT FAQs
Interactive Response Technology (IRT) is a term for clinical trial management software that handles patient randomization, drug assignment, and supply chain logistics.
IRT automates critical processes like patient enrollment, randomization, treatment allocation, inventory tracking, and compliance monitoring that were traditionally handled manually or through phone systems.
Core IRT functions:
- Patient management: Screening, enrollment, randomization, and visit schedules
- Drug supply management: Inventory tracking, automated resupply, and distribution optimization
- Data integration: Real-time reporting and seamless integration with other clinical systems
IRT systems from vendors like Suvoda provide web-based interfaces with advanced configurability to handle complex protocol requirements across all trial phases.
In today’s trials, the two terms are often used interchangeably. Most modern IRT systems, including Suvoda IRT, deliver full RTSM functionality.
Interactive Response Technology (IRT) refers to the technology system clinical trials use to manage key trial activities such as randomizing patients, managing drug supply and patient visits. "Interactive response" refers to how the software allows users (like site staff) to interact with the system in real time. This is the broader term.
Randomization and Trial Supply Management (RTSM) describes the specific functions the IRT system performs, like assigning participants to treatment arms and managing the flow of investigational product.
So, RTSM is what the system does, while IRT is the technology that makes it possible.
Historically, IRT described systems that allowed users like site staff, monitors, study teams to interact with trial workflows through IVRS (phone), IWRS (web), or later integrated digital interfaces. The emphasis was on the mode of interaction, not the function.
Suvoda stands out for being part of a unified platform, its Sofia AI assistant, and extensive configurability for complex studies. Unlike standalone IRT vendors, Suvoda's platform approach combines IRT with eConsent, eCOA, Patient Payments, Patient Travel, Scheduling, and the Greenphire Clinical Finance suite on a single data model.
Suvoda IRT’s key differentiators:
- Unified platform: Single data model across all products on the Suvoda Platform
- Sofia AI assistant: Conversational interface for drug supply management
- Complex study expertise: Specialized in oncology, CNS, and rare disease trials
- Modular, patented architecture: Rapid deployment and mid-study modifications
- Self-service capabilities: Reduce dependency on vendor for routine changes
This approach eliminates traditional system silos and reduces integration complexity for sponsors managing multiple eClinical technologies.
Powering the patient's digital journey in life-changing clinical trials
Broad perspective. Keen insight. Calm guidance.
Broad perspective. Keen insight. Calm guidance.
Seamless technology in complex ecosystems
Seamless technology in complex ecosystems
INSIGHTS & NEWS
-
BLOG
What patients are asking for in clinical trials, according to the CISCRP 2025 survey
Feb 26, 2026 -
NEWS
Suvoda advances clinical trial financial planning with support for complex multi-payee budgets
Feb 25, 2026 -
BLOG
IRT FAQ: A guide to interactive response technology for clinical trials
Feb 23, 2026 -
CASE STUDIES & REPORTS
Choosing an eCOA partner you can trust starts here
Feb 17, 2026 -
CASE STUDIES & REPORTS
Discover what patients are telling us about clinical trials
Feb 17, 2026 -
BLOG
Clinical trial technology 2026 outlook: five big ideas from Suvoda leaders
Feb 12, 2026 -
BLOG
A conversation with E.K. Koh, Chief Product Officer: how Suvoda is shaping the future of clinical trial technology
Jan 29, 2026 -
NEWS
Suvoda sets new standard for streamlined patient and site experience in clinical trials
Jan 12, 2026 -
BLOG
Less friction, more focus: Rethinking technology for patients and sites in clinical trials
Jan 8, 2026 -
BLOG
What the Suvoda-Greenphire merger means for clinical trials
Dec 18, 2025