Suvoda eCOA Buyer's eBook
Electronic Clinical Outcome Assessment (eCOA) software plays a central role in clinical trials, shaping the experience for patients, sites, and sponsors alike. Yet many sponsors still struggle to find the right eCOA solution that meets their needs. Delays, poor user experience, lack of flexibility, difficulties with global implementation, and data reconciliation challenges continue to be felt by the industry.
As trials grow more complex and the pressure to deliver results intensifies, CROs, sites and sponsors need an eCOA solution that does more than collect data. They need to create smoother, more engaging, and more compliant study experiences for everyone involved.
Suvoda eCOA was designed with that goal in mind. Built on the Suvoda Platform, it works natively across Suvoda solutions with no integration required, while also connecting easily to other clinical technologies through its API layer to support global, end-to-end trial management in complex real-world environments. However, technology alone isn’t enough. The right provider must also bring therapeutic expertise, collaborative partnership, and operational excellence to truly make a difference.
This guide shares what we’ve learned to help sponsors make confident, informed choices when evaluating eCOA providers. We explore five common challenges sponsors face today, and how Suvoda tackled each one to deliver a purpose-built eCOA solution that strengthens engagement, improves data quality, supports compliance, and empowers sites throughout the trial lifecycle.
Table of contents
-
Legacy eCOA pain points
-
Challenge 1: Unpredictable implementation timelines and delayed study startups
-
Challenge 2: Data reconciliation challenges across systems
-
Challenge 3: Disconnected point solutions that don’t adapt to changes
-
Challenge 4: Regulatory submission delays for global studies
-
Challenge 5: Frustrating and disconnected site user experience
Suvoda eCOA: Purpose-designed |
|
|
|
|
|
Challenge 1
Unpredictable implementation timelines and delayed study startups
THE SUVODA SOLUTION: Reliable, on-time study deployment and delivery
Suvoda teams ask the right questions in order to build efficient and reliable timelines that help sponsors launch on schedule without compromising quality or compliance. In addition, Suvoda eCOA questionnaire development is separated from the overall study build, allowing workstreams to advance independently and simultaneously.
Reliable timelines and expert, global service teams
Protocol, licensing, and localization experts are involved even before kickoff and throughout implementation, acting as consultative partners for the life of the trial to keep it on track.
Rapid build and testing cycles
With a build process that is agile and that adjusts as needs evolve, teams are able to reach go-live on time.
Patented questionnaire design language
The Suvoda Questionnaire Definition Language (SQDL) was awarded a U.S. patent for its innovative approach and allows questionnaire creation, translation, and layout work to proceed concurrently, reducing timelines from weeks to days.
Modular questionnaire repository
Reusable, pre-built diaries and questionnaires help reduce design and build time across studies.
![]() Delayed system go-live is one of the most common complaints about legacy eCOA systems. Sponsors may hear idealistic timelines early in the process, only to see dates shift as implementation progresses— eroding trust and making it harder to plan with confidence.
Delayed system go-live is one of the most common complaints about legacy eCOA systems. Sponsors may hear idealistic timelines early in the process, only to see dates shift as implementation progresses— eroding trust and making it harder to plan with confidence.
One core aspect of eCOA solutions— questionnaire development—can become a bottleneck when teams must wait for one step to complete before beginning the next. When that happens, build, testing, and translations cannot be done in parallel, creating a list of setbacks that push study start dates further out.
"One of the greatest challenges we’ve had with eCOA partners is transparency with timelines and achieving those timelines."
— David Vale,
Senior Director,
Program Management,
Cara Therapeutics, Inc.
Challenge 2
Data reconciliation challenges across systems
Some of the biggest data reconciliation challenges with eCOA stem from system fragmentation, manual processes, and human error, which can lead to downstream data management headaches both mid-study and at study closeout.
THE SUVODA SOLUTION: Less manual entry and fewer errors with passive data capture
Suvoda eCOA was intentionally built on the Suvoda Platform with a shared back-end architecture. This approach passively captures key study data as users engage with the system—including visit confirmations, labels, dates, and times—reducing the reliance on manual entry that often introduces errors and downstream corrections.
![]() On average, Suvoda trials generate fewer than 10 data change forms compared to the hundreds per trial typically seen in the industry, so sponsors and sites spend less time correcting data and more time focusing on patients. For sponsors, this translates into fewer costly delays and greater confidence in trial outcomes.
On average, Suvoda trials generate fewer than 10 data change forms compared to the hundreds per trial typically seen in the industry, so sponsors and sites spend less time correcting data and more time focusing on patients. For sponsors, this translates into fewer costly delays and greater confidence in trial outcomes.
Guided workflows |
Seamless syncing |
Focused, high-quality data capture |
Built-in engagement & compliance features |
|
Protocol-aligned steps that reduce or eliminate common mistakes, such as confirming future visits or skipping required visits, reduce the need for post-entry corrections and data change forms (DCFs). |
Real-time data sharing reduces redundant data entry for improved efficiency and data consistency. For example, when Suvoda eCOA is paired with Suvoda IRT, data in one system is instantly available in both solutions. No more waiting for an EDC to sync.
|
Tailored workflows that collect only necessary data, reduce noise and protocol deviations for cleaner, more submission-ready datasets. |
Intuitive interfaces, reminders, and usability across patients and their caregivers support timely, complete data entry and sustained participant engagement. |
Challenge 3
Disconnected point solutions that don’t adapt to changes
THE SUVODA SOLUTION: Built for adaptability and interoperability
Suvoda eCOA delivers the technical flexibility to adapt to each protocol and the interoperability to connect seamlessly with other solutions on the Suvoda Platform, reducing operational friction for sites and sponsors alike.
Flexible, modular architecture
The software can adapt mid-study to support protocol amendments or the addition of new countries and languages without requiring a full system rebuild and revalidation.
Scalable and extensible
The platform supports studies ranging from small, single-site trials to large, multinational trials with complex requirements.
Device and deployment flexibility
Teams can choose from BYOD, web app, provisioned device, and hybrid models, with global logistics expertise supporting timely delivery.
Outcomes data is made available in real time
eCOA data is immediately available to other workflows on the Suvoda Platform, supporting time-sensitive decisions such as dose adjustments during on-site visits.
Automated eligibility gating
The platform prevents ineligible participant randomization by using eCOA outcomes data as eligibility criteria within Suvoda IRT.
Trigger-based automation
Conditional workflows can initiate actions such as patient payments upon questionnaire completion
![]() In clinical trials, it’s common for requirements to shift mid-study. New sites may be added or challenges with patient enrollment A systems can’t easily adjust once configured. They often feel rigid, forcing teams to adapt protocols to the software instead of the other way around. This one-size-fits-all approach can slow timelines and limit the ability to support complex or adaptive study designs.
In clinical trials, it’s common for requirements to shift mid-study. New sites may be added or challenges with patient enrollment A systems can’t easily adjust once configured. They often feel rigid, forcing teams to adapt protocols to the software instead of the other way around. This one-size-fits-all approach can slow timelines and limit the ability to support complex or adaptive study designs.
Additionally, many legacy eCOA solutions operate in silos, disconnected from critical systems like IRT, eConsent, or Patient Payments. These gaps can create redundant work, cause delays in time-sensitive decisions, and add even more friction during mid-study updates.
Challenge 4
Regulatory submission delays for global studies
Many eCOA providers struggle to manage questionnaire localization and the associated timelines, introducing risk and delaying trials. Without in-house expertise and disciplined localization project management for global studies, localization requirements can quickly become bottlenecks that delay study start dates.
THE SUVODA SOLUTION: Global trials supported by expert licensing and localization services
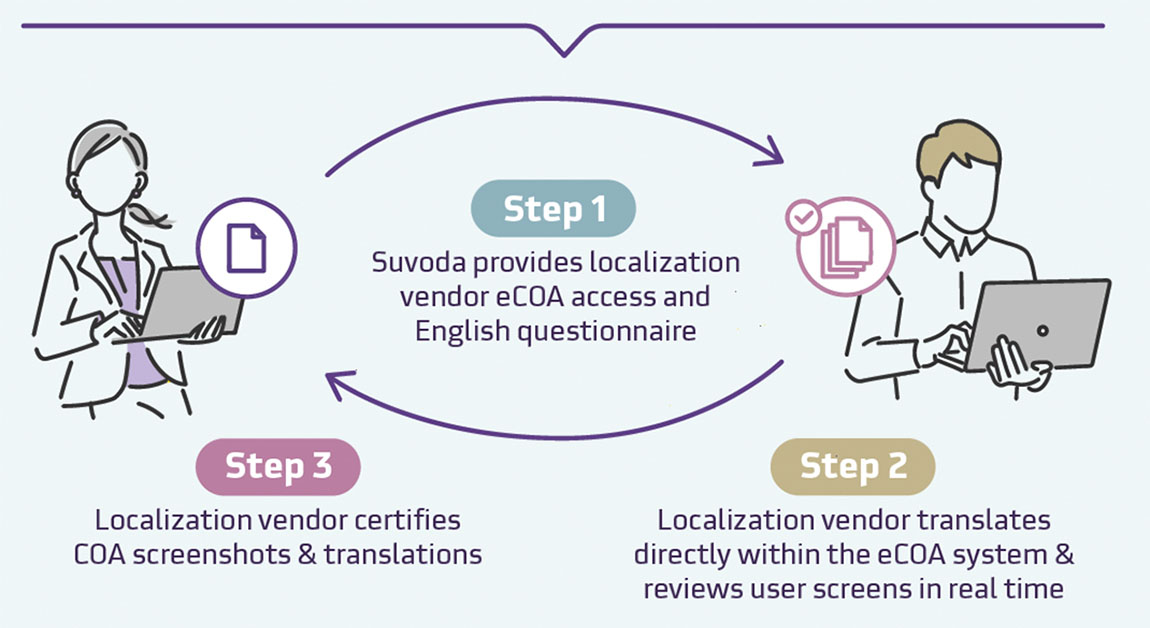
Suvoda’s in-house experts and the Suvoda 3-step localization process help manage questionnaire licensing, translation, and localization from start to finish, reducing handoffs and preventing common pitfalls that derail timelines.
Real-time review
Ongoing visibility into translation and localization progress helps maintain accuracy and transparency.
Comprehensive localization process
In-house experts oversee translation, back-translation, and cognitive debriefing to keep timelines on track.
SUVODA'S 3-STEP LOCALIZATION PROCESS
Challenge 5
Frustrating and disconnected site user experience
THE SUVODA SOLUTION: User-centered design supported by a unified eClinical ecosystem
Suvoda eCOA is built with practical, user-centered design principles to empower the people who make life-changing studies happen. Delivered as a standalone product, or better, on the Suvoda Platform with a unified suite of solutions, Suvoda eCOA maintains its accessibility through a familiar, intuitive interface.
Sites are guided by protocol-aligned workflows, and users stay engaged. Data, tasks, and activities are synchronized. The result is clarity, confidence, and a seamless experience for everyone involved.
The Suvoda Platform delivers:
Single, robust data layer and sign-on
Unified data architecture supports transparency, consistency, fast access, and improved integrity across all platform components.
Unified patient app
A single interface consolidates questionnaires, visit scheduling, travel management, and payments to reduce friction and improve compliance.
Streamlined navigation
Users can access what they need with a friendly dashboard of relevant solutions.
Real-time data capture with smart reminders
Assessments and diaries are accessible anywhere and are supported by timely notifications that reduce “parking-lot” backfilling and improve data accuracy across diverse patient populations.
![]() Many eCOA solutions emphasize technology over people, resulting in disconnected, hard-to-use systems that disrupt workflows and erode engagement. When eCOA isn’t designed around the people who use it—patients, sites, and sponsors—users may disengage and trials may falter.
Many eCOA solutions emphasize technology over people, resulting in disconnected, hard-to-use systems that disrupt workflows and erode engagement. When eCOA isn’t designed around the people who use it—patients, sites, and sponsors—users may disengage and trials may falter.
This is especially true for eCOA solutions that do not readily communicate with other solutions, which makes it difficult for study teams to determine next steps in a workflow. Point solutions can also require repeated manual entry of core study information like participant IDs, introducing errors and adding to frustration.
“Suvoda eCOA was incredibly easy for our patients to use. We had an increase in patient compliance in completing PRO questionnaires, as compared to previous studies with other eCOA providers.”
— Catherine Munera, Vice President of Biometrics, Cara Therapeutics
What to look for in your next eCOA solution
Choosing an eCOA solution is ultimately about confidence. Sponsors need to know that timelines will be met, data will be reliable, global requirements will be handled correctly, and patients and sites will be supported without unnecessary complexity. Too often, gaps in these areas only become visible once a study is already underway.
The five criteria outlined in this guide provide a practical framework for evaluating whether an eCOA provider can deliver on what they promise. Predictable implementation, purpose-built data capture, protocol-driven flexibility, global localization expertise, and a connected, user-centered experience are not just technical capabilities—they are indicators of transparency, operational maturity, and dependable partnership.
Suvoda eCOA was built with these principles at its core. Delivered on the unified Suvoda Platform, it brings together technology, expertise, and services designed to reduce risk, support informed decision-making, and keep studies on track. The result is an eCOA solution sponsors can trust to perform consistently—from startup through study close.
Suvoda’s north star is clear: keep life-changing clinical trials moving forward to advance human health. That commitment shapes how we design and deliver Suvoda eCOA on the unified Suvoda Platform, creating a seamless, connected experience that supports patients, sites, and sponsors at every step of a clinical trial.
Key criteria |
Legacy eCOA |
Suvoda eCOA |
|
Predictable, transparent implementation timelines |
No |
Yes |
|
Reduced data reconciliation headaches |
No |
Yes |
|
Protocol-driven flexibility |
No |
Yes |
|
Confidence in localization delivery timelines |
No |
Yes |
|
Connected cross-platform experience to guide patients, sites, and sponsors |
No |
Yes |