Originally published on the Greenphire website prior to the merger with Suvoda in 2025.
Snapshot:
Expanded platform, broader impact: Greenphire’s financial and logistical solutions are joining the Suvoda Platform, offering more comprehensive support for global trials.
Streamlined trial execution: The combined offering connects patient payments, data collection, supply management, consent workflows, and more on a unified system.
One partner for end-to-end support: Sponsors, CROs, sites, and patients benefit from integrated tools designed to reduce burden, improve coordination, and simplify operations.
For over 15 years, Greenphire has been connecting the dots across disparate processes and stakeholders to support global clinical trials and their patients. From participant reimbursements and travel coordination to study budgeting and site payments, our solutions have been designed to remove the financial and logistical barriers that can derail clinical trials and impact patient access to innovative therapies.
Now, through our completed merger with Suvoda, we’re expanding those capabilities, but with the same shared commitment to reduce the burdens on clinical trial patients, sites, sponsors, and CROs.
Get to know the Suvoda Platform and solutions
We’re excited to be bringing Greenphire’s solutions to the Suvoda Platform, which provides a suite of best-in-class eClinical software products. Together, we’ll be able to provide end-to-end support to your trials.
Suvoda specializes in managing the most urgent moments in complex, life-sustaining global studies. The platform’s solutions address the operational challenges that have traditionally made trials difficult to execute and complete successfully.
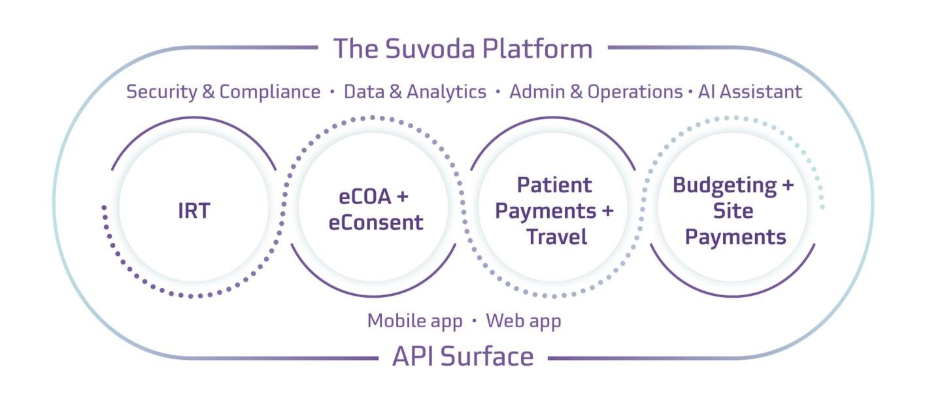
If you haven’t explored Suvoda’s products before, here’s a quick snapshot:
- Suvoda IRT (Interactive Response Technology): Sophisticated randomization and drug supply management system that ensures the right drug reaches the right patient at the right time, featuring Sofia AI assistant for busy drug supply managers.
- Suvoda eCOA (Electronic Clinical Outcome Assessment): Purpose-built patient outcomes data collection solution with patented questionnaire design technology that accelerates deployment. High-quality data collection supports regulatory submissions and clinical decision-making.
- Suvoda eConsent: Electronic informed consent solution that enhances patient comprehension through interactive features while providing complete visibility over consent status across all study sites, reducing regulatory risks and potential study delays.
Streamlining the complete clinical trial journey
This merger provides more than just expanded service offerings. Where we’ve excelled at removing financial barriers and logistical obstacles, Suvoda brings the operational expertise to address the complex data management and supply chain challenges that can make or break a study.
The combined platform will originally offer eight solutions and enable sponsors and CROs to manage their trials through a unified system that connects financial processes, patient engagement, and clinical operations. Instead of juggling multiple vendors and systems, research teams can work through integrated workflows that maintain data integrity while simplifying day-to-day trial management.
What this can mean for your trials
This creates an opportunity to significantly reduce the disconnected processes that have traditionally complicated clinical research. Sites can manage patient payments, travel, and invoicing alongside clinical data collection. Sponsors gain unified visibility into both trial finances and operational performance. Patients experience fewer technical systems and smoother coordination across all aspects of their trial participation.
As we move forward, the combined organization will operate under the Suvoda name, but the Greenphire commitment to making clinical trials GO remains unchanged. We’re simply expanding our ability to connect the dots across an even broader range of clinical trial processes and stakeholder needs.
For research teams looking to streamline their operations while maintaining the highest standards of patient engagement and data quality, this expanded platform represents a new level of integrated support. The future of clinical trials lies in seamlessly connected solutions that address every aspect of the research process and patient journey.
What’s next?
Customers will begin receiving updates and information about product developments and opportunities. Next month, our websites and social media will be merged under the Suvoda brand. Please be sure to follow Suvoda on LinkedIn, and bookmark suvoda.com for the latest news and information.
Ready to explore what the Suvoda Platform can do for your studies? Contact us to learn more about how our enhanced capabilities can help make your next trial more efficient, patient-friendly, and successful.
