Author: Heather Nonnemacher, Associate Director, Services Solutions
Snapshot:
- AI’s Growing Influence: AI technologies are beginning to transform the drug development processes, with significant investments across the pharmaceutical and biotechnology industries.
- Cautious Optimism: Measured enthusiasm for AI in clinical trials is on the rise, recognizing the need for additional testing and ensuring human oversight.
- Progressive Integration: A phased application of AI in clinical trial processes has the potential to streamline operations, enhance data analysis, and shorten trial timelines, ultimately accelerating the delivery of life-saving medicine to patients.
AI as an emerging mainstream technology
The public’s awareness and acceptance of Artificial Intelligence (AI) reached new heights in 2023. This is largely due to ChatGPT’s emergence into the mainstream, and it appears to be ushering in a new era of technological possibility. Like in most industries, we are beginning to understand the significant implications for AI use in drug development and clinical trials.
For example, the number of AI-driven firms specializing in drug discovery and development has expanded dramatically, from 62 in 2011 to 400 firms in 2022.1,2 Leaders in pharmaceuticals and biotechnology, such as Roche, Novo Nordisk, and Sanofi, are increasingly leveraging AI to streamline drug discovery processes and enhance patient outcomes.
Roche’s generative AI tool, RocheGPT, augments analysts’ efforts by analyzing scientific articles or clinical test results and then extracting structured data about therapies and patients.3 Similarly, Novo Nordisk’s establishment of an AI-based research facility for the discovery and development of drug candidates and Sanofi’s utilization of AI platforms for developing small molecule candidates spotlight the growing adoption of AI technologies.4,5
A recent Suvoda LinkedIn poll aligns with these developments; we found that 63% of our clinical trial community believed AI was the technological trend that would dominate the market in 2024.
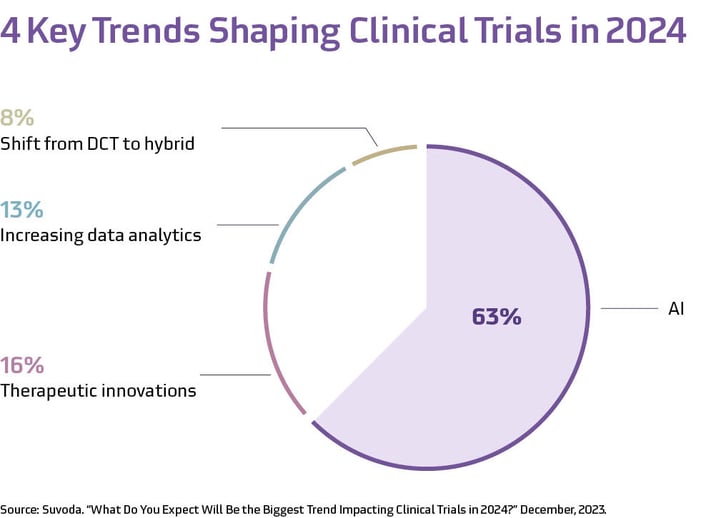
It’s clear that AI represents an important advancement with potential to push the clinical research industry forward. However, because our work intersects with patients and their health, taking a balanced approach focused on safety, ethics, privacy and data security, and complementing human effort is crucial to maximize AI’s potential benefits.
The foundation for strategic AI use in clinical trials
Many within the clinical trial industry appear to hold a similar practical perspective. Another LinkedIn poll revealed a cautious optimism towards the use of AI in clinical trials. Despite excitement about AI’s potential, more than half of poll respondents also want to see further learning and testing.
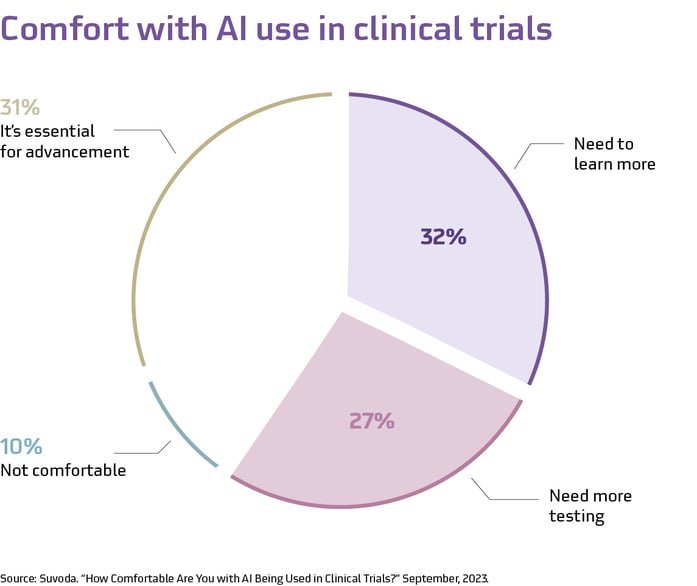
At present, AI is already helping to streamline some processes in clinical trials. However, it is not perfect. When looking at large language models (LLM), for example, AI may return inaccurate data or misinterpret nuances in informed consent documents or clinical trial protocols, emphasizing the need for human oversight, especially at its current stage of maturity. AI is best used to augment human work, rather than as an independent or comprehensive solution, and it is critical to have human review factored into any process AI is supporting.
Clinical researchers looking to deploy AI in their daily workflow can do so by setting a solid foundation for practical, strategic use. For example:
- Leveraging AI as a tool to support human efforts. Identify areas to deploy AI to help people be more efficient in their work.
- Integrating AI with existing clinical trial technology. AI has the potential to work as an augmentative tool, making existing technology more user-friendly.
- Prioritizing data security and compliance. Adhering to stringent data security measures and compliance with regulatory standards can help foster trust in AI-powered solutions.
- Engaging in collaborative development. Ongoing collaboration among AI developers, researchers, clinicians, and ethicists will ensure AI’s responsible and productive deployment, especially in urgent clinical scenarios.
AI to remove friction from the clinical trial journey
Suvoda is exploring a phased introduction of AI, carefully testing its potential to refine our processes. While it’s not yet a frontline tool for patient or site interactions, our iterative testing and learning approach is a priority for Suvoda leadership and paving the way toward broader application. We are considering where AI might be applied to support processes before the trial begins (e.g., protocol design, building and testing trial software), during the trial (e.g., customer support, patient comprehension, drug supply management), and after the trial (e.g., facilitating data analysis, participant support).
The use of AI in clinical trials to date suggests an emerging framework for its role in the industry. From compound development and strategy to clinical trial execution, AI could be shortening each trial phase, potentially contributing to bringing life-saving medicines to the market more quickly. Despite its nascent stage, the rapid evolution of AI in clinical trials is undeniable.
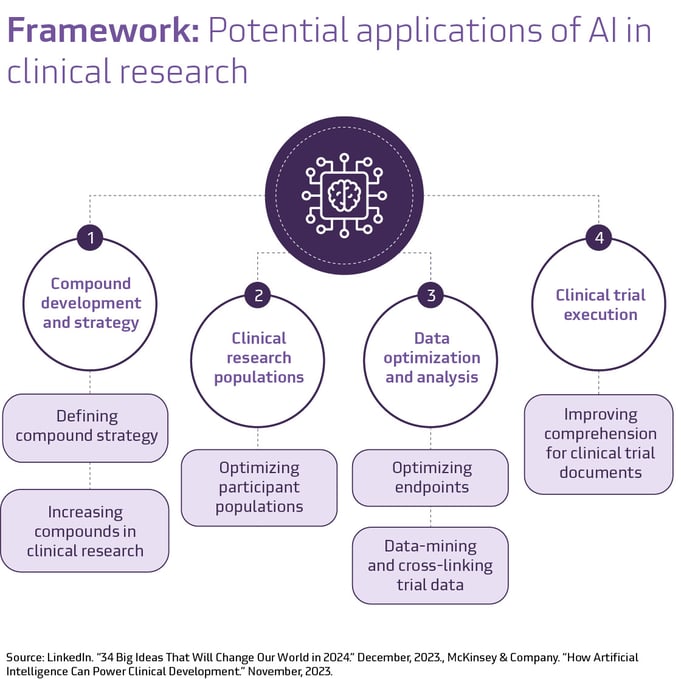
The future of AI in transforming clinical trials
AI’s capacity for automating processes, enhancing data analysis, and streamlining procedures will undoubtedly facilitate more efficient and innovative practices among scientific, clinical, and technological teams.
By pragmatically applying AI in stages, we have an opportunity to reduce friction in mission-critical, urgent moments of clinical trials. It may be one more important tool to support sponsors, sites, and patients in our collective efforts to bring medicines to those who need them.
Understand how AI is shaping clinical trials.
Learn about the adoption and impact of eConsent, IRT (Interactive Response Technology), eCOA (Electronic Clinical Outcome Assessments), and AI (Artificial Intelligence) within the clinical trials sector.
• Increasing Technology Integration
• Benefits for Sponsors and Patients
• Future Potential of AI
References
- “12 Notable AI-Powered Biotech Companies Founded in 2021,” BioPharmaTrend, June 23, 2023, https://www.biopharmatrend.com/post/500-10-notable-ai-powered-biotech-companies-founded-in-2021/.
- “AIi’s Pivotal Role in Drug Discovery and Development in 2023,” Drug Discovery and Development, October 23, 2023, https://www.drugdiscoverytrends.com/ai-drug-discovery-2023-trends/.
- “Roche GPT: A Generative AI Tool for Healthcare Data Protection.: David Drodge Posted on the Topic,” LinkedIn, September 24, 2023, https://www.linkedin.com/posts/davedrodge_chatgpt-health-rochegpt-activity-7111805972209090561-ATUk/.
- “Novo Nordisk to Open New AI Hub in UK for Drug Discovery,” PharmaTimes, January 3, 2024, https://pharmatimes.com/news/novo-nordisk-to-open-new-ai-hub-in-uk-for-drug-discovery/.
- “How French Pharma Giant Sanofi Is Betting Big on Ai,” Labiotech, November 2, 2023, https://www.labiotech.eu/in-depth/sanofi-ai-deals/.
Author

Heather Nonnemacher
Associate Director, Services Solutions, Suvoda

.png?width=303&height=362&name=Screenshot%202024-09-13%20at%202.25.54%20PM%20(2).png)