By: Marc Lisi, Co-Founder and Director, Customer Solutions
- Flexible Drug Management: Drug pooling is a supply chain methodology where drug units are managed in a centralized supply at the depot level and can be available for ordering across multiple clinical trials. This allows drug units to be assigned to an inventory pool rather than a single protocol.
- Strategic Supply Planning: Interactive Response Technology (IRT) can enable sponsors to manage drug pooling effectively by allowing drug types, depots, lots, and country-specific label groups to be shared across trials.
- Cost Reduction and Waste Minimization: Drug pooling can minimize waste and improve efficiency in delivering drugs to patients, although it may not be suitable for every sponsor, study, or product. It should be considered during trial planning to optimize clinical supply strategies.
Drug pooling has long been a topic of conversation among clinical supply managers. In 2013, the year Suvoda was founded, both vendors and sponsors were talking about it. In fact, about 20 percent of all the sessions at a three-day industry conference that year were related to pooling inventory. Drug pooling seemed to be on everyone’s mind.
Fast forward over a decade, the use of drug pooling is far less common than expected, given the level of attention it received. While some sponsors and clinical supply managers swear by it, many across the industry agree that drug pooling has not taken off like we expected it would back in 2013.
So, what happened to drug pooling? Does it still play a role in today’s increasingly complex trials? The answer is, yes. Despite lower adoption levels, clinical trial design getting more and more complex, time pressures to deliver on these trials continually increasing, and sponsors looking to save costs wherever possible, drug pooling can prove to be extremely beneficial in specific situations.
Drug Pooling 101
Let’s start with the basics – what is drug pooling? Drug pooling is a supply chain methodology where drug units are managed in a centralized supply at the depot level and can be available for ordering across multiple clinical trials. When using drug pooling, drug units do not need to be associated with a single protocol, but instead can be assigned to an inventory pool which can supply multiple studies. This works best when the drug product is packaged the same way and is being used in a consistent manner across all trials in the pool.
From a technical perspective, IRT can enable sponsors to closely manage drug pooling. Drug types, depots, lots, and country-specific label groups may be shared across trials, both for serialized and non-serialized investigational product or ancillary supplies. In the traditional IRT model, Drug Unit 12345 has always been assigned to Protocol ABC-101. In a pooled model, however, Drug Unit 12345 is assigned to Protocol ABC-101 in the IRT at the time drug is ordered. Once a drug order is generated by the IRT system, a drug order form is emailed to the depot and simultaneously automatically imported directly into the depot’s ERP system. This drug order form specifies the protocol number, which allows the depot to perform “just in time” labeling, if required.
Flexibility for the Unexpected
We all like to think clinical trials will run as planned, but in reality they rarely do. Some trials will enroll much faster than expected, while others inevitably enroll much slower. Drug pooling provides flexibility to accommodate these different scenarios.
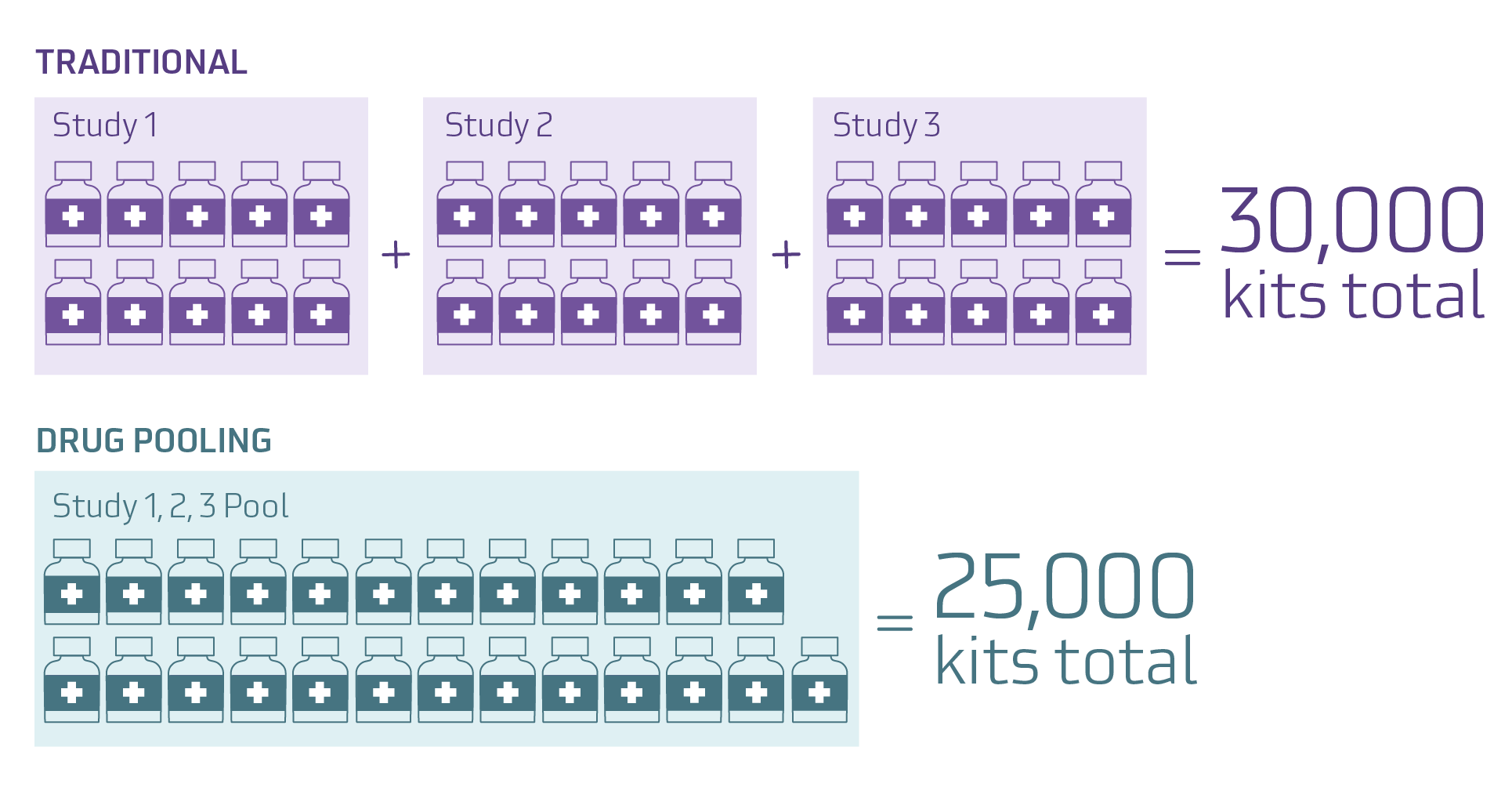
In the real-world example above, Study 1 only uses 7,500 kits, Study 2 has enrollment increased and uses 12,000 kits, and Study 3 is canceled early and only uses 4,000 kits. By utilizing drug pooling the sponsor saves packaging and labeling costs for 5,000 kits, and also avoids packaging and labeling an additional 2,000 kits for Study 2.
The benefit of drug pooling is clear – it can reduce overage and waste. Reduced waste leads to cost savings, which is more important today than ever. Rather than packaging and labeling 10,000 kits for each of three studies, the sponsor was able to choose to package and label 25,000 kits for the program. This flexibility is a significant advantage for supply planning.
Considerations for implementing drug pooling
While the benefits are clear, there are of course considerations with this approach, including:
- Identifying and selecting IRT and packaging/distribution vendors who can support drug pooling.
- Engaging in earlier supply planning discussions that consider label requirements and design, order processing, and kit release procedures at the pipeline level instead of at the study level.
- Making decisions for Study #1 that will have downstream effects on Study #5.
While these considerations may require a mindset shift within an organization, these adjustments will open up the door to bring more flexibility to clinical supply strategies.
Although drug pooling may not be the right choice for every sponsor, for every study, or for every product, it is important that it remains a consideration during trial planning. For those clinical trials where drug pooling makes sense, waste can be minimized and drugs can be delivered more efficiently to those patients who need it most.
Learn how Suvoda’s flexible approach to IRT can help your trial.
Explore how Suvoda's IRT helps manage multi-drug dosing efficiently.
Learn how a biotech leveraged Suvoda IRT to enable:
• Real-time visibility and control over complex drug dispensation scenarios and visit schedules
• High-quality data integrity with accurate drug dispensation tracking
• Minimized disruptions and reduced delays from protocol modifications
Author

Marc Lisi
Co-Founder and Director of Customer Solutions
Suvoda
Marc has spent his entire career in the eClinical technologies space, with most of that time focused in the area of IRT. Having spent years on the client services side, Marc has worked with 7 of the top 10 pharmaceutical companies and 6 of the top 7 CROs to design, implement, and maintain IRT solutions across a variety of therapeutic areas.

.png?width=265&height=342&name=Screenshot%202024-09-13%20at%209.13.54%20AM%20(2).png)