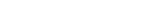A centralized control center for patient and drug logistics, designed to harness the complexities of clinical trials and help advance human health.
Managing clinical trial randomization and supply logistics can be challenging, especially with the complexities of modern clinical trials. Hybrid trial approaches, tight timelines, and the need for mid-study adjustments mean that sponsors and sites need precise and powerful systems to keep trials running efficiently and on-time.
From initial setup to mid-study adjustments to study completion, Suvoda Interactive Response Technology (IRT) gets the right drug to the right patient at the right time, with advanced randomization and drug supply management capabilities that integrate seamlessly with existing clinical workflows and eClinical systems.
Now with Sofia, Suvoda’s safe and secure AI assistant, drug supply managers can have efficient access to vital information through an intuitive chat interface, reducing what were once multi-screen, multi-click processes to simple conversations. Sofia is currently in the early adopter stage for customers that wish to enable the technology. We welcome sponsors and CROs to join us to further refine its capabilities. To learn more about how Sofia works, please see our FAQs.
Learn more about Sofia Early Adopter Program
Suvoda IRT keeps trials running smoothly and supports sites, sponsors and CROs to:
- Manage all clinical trial workflows efficiently, ensuring data is shared across systems
- Meet exact trial randomization and drug supply needs with extensive configurability and customizability
- More simply conduct patient visits and drug dispensation with an intuitive interface and user-centric design
- Access robust, real-time reporting for enhanced trial oversight and decision-making
Clinical trial complexity, meet our rich IRT functionality. Now you can address unique and changing needs—and hit tight timelines.
A robust foundation of essential core IRT features, each focused on meeting the requirements of common protocols in decentralized, patient-centric trials. The tools to create new, client-custom functionality to support clinical research of increasingly novel, life-changing therapeutics. All developed as modular, easy-to-assemble building blocks. So our services teams can quickly launch systems that embrace the highest levels of protocol, operational, logistical, technical, and cultural complexity.
What’s more, the Suvoda IRT system also includes extensive configurability options. It’s how we enable study teams to adjust system functionality over the lifespan of a trial to meet ever-changing demands. Because we know that no two of our clients’ trials start out exactly alike. We also know trials need to evolve, with mid-project course corrections to respond to the specific hurdles they encounter or insights they collect. And they all need to do so with speed and ease.
Trial Logistics
-
Roles, permissions, and blinding management
-
Study and site administration
-
Cohort/stage/phase management
-
Dynamic cohort and dose management
-
Additional and dynamic visit schedule
-
Embedded, intuitive Sofia AI assistant
Patient Logistics
-
Subject management
-
Adaptive replacement and randomization
-
Cross-over and re-treatment
-
Open-label extension
-
Dose calculation
-
Subject roll-over
-
Dose modification and interruption
Drug Logistics
-
Drug dispensing management
-
Drug supply management
-
Drug accountability, reconciliation, and returns/destruction management
-
Temperature excursion management
-
Controlled substance management
-
Variable drug sourcing
-
Central pharmacy
-
Direct-to-patient shipping
-
Robust supply strategy management
-
Fast access to drug supply data and visualizations with Sofia AI assistant
-
Drug supply forecasting
Built to embrace the unknown. Because your IRT should be as ready for change as you are.
The scientific process isn’t static. And neither is your trial. To collect the richest data, protocols must be amended as new information is obtained. That’s why we designed the Suvoda IRT to adapt mid-study with ease.
Commonly-needed additions, modifications, and corrections to study, site, drug management, and administrative functions can be made by permissioned users within our system after go-live. This puts control in your hands and reduces the time and costs associated with change-orders. And if more significant updates are required, our modular architecture makes it a simple and speedy process for your dedicated Suvoda project team to add in new IRT features.
A user-friendly IRT experience eases decisive action for users of all experience levels.
We find that people are more likely to work at their best when they’re using the tools they like best.
That’s why, no matter the level of experience with clinical trial technology—from dedicated pros to more occasional site-users, and even home caregivers—you’ll find interactions with the Suvoda IRT are universally simple. It’s the result of a minimalist look and feel, intuitive navigation, and patient-centric workflow. And it’s all intended to drive focus on the immediate task at hand.
Tasks are now even faster and easier with Sofia, Suvoda’s AI assistant. Users can have the information they need at their fingertips just by asking Sofia questions. They can access trial data, look up user manual topics, compare depot inventories, review drug lot releases, create tables and graphs, and more, all while maintaining the study blind and with assurance that the answers are accurate. To learn more about how Sofia works, please see our FAQs.
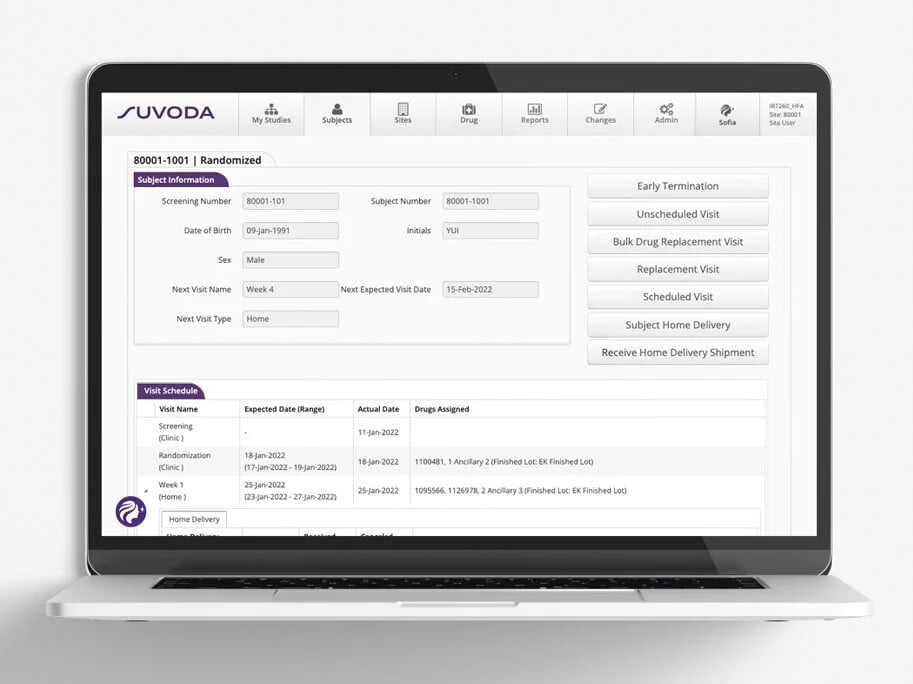
See the way to long-term trial performance, in real time, with pre-set and ad-hoc reports and advanced analytics.
Suvoda IRT pre-set and ad-hoc reports give you up-to-the-minute visualizations of data points, KPIs, and trends on the subjects, sites, drugs, and depots within a given study. It’s how you’ll always know where you stand—and what needs your attention.
Why such robust reporting? To give you the intelligence you need to make better decisions in executing on today’s trials—and planning for tomorrow’s.
FEATURED IRT MODULES

Take command of the cold supply chain
Gain control in a world of decentralized trials
Gain control in a world of decentralized trials
Broad perspective. Keen insight. Calm guidance.
Broad perspective. Keen insight. Calm guidance.
Seamless technology in complex ecosystems
Seamless technology in complex ecosystems
INSIGHTS & NEWS
-
BLOG
Honoring patient altruism: empowering clinical trial participants with better technology
Jul 10, 2025 -
BLOG
Sustainable clinical trials through eClinical innovation
Jun 19, 2025 -
BLOG
How Suvoda is driving innovation in clinical trial software: 3 trends you need to watch
Jun 5, 2025 -
BLOG
Innovation with safeguards: managing risk and regulation in clinical trial technology
May 22, 2025 -
NEWS
Suvoda and Greenphire Announce Completion of Merger
Apr 24, 2025 -
BLOG
Q&A: What do the recent updates to the Declaration of Helsinki mean for clinical trial technology?
Apr 10, 2025 -
CASE STUDY
Unraveling the complexity of oncology trials: insights into the potential of eClinical solutions
Apr 3, 2025 -
BLOG
Why eCOA belongs with IRT: connected data for mission-critical, time-sensitive moments in a clinical trial
Apr 3, 2025 -
BLOG
The Suvoda Platform: innovation and architecture that delivers simplicity and customizability
Mar 27, 2025 -
ON-DEMAND
Applying technology advancements to overcome challenges in clinical trials
Mar 20, 2025